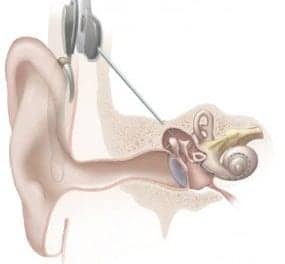
Cochlear Limited (ASX:COH) announced the US Food and Drug Administration (FDA) approval of three new products to its suite of hearing technology solutions. The approval of the CochlearTM Nucleus® Kanso® 2 Sound Processor, Nucleus® 7 Sound Processor for Nucleus 22 Implant recipients, and Custom Sound® Pro fitting software reflects Cochlear’s “ongoing commitment to innovation in hearing technology, providing access to smartphone connectivity and helping to improve hearing performance, and enhancing the cochlear implant fitting experience for hearing health professionals.”
“Cochlear continues to develop and introduce products to support the best lifelong hearing experience for our recipients and their care team,” said Tony Manna, President, Cochlear Americas. “Our new technology can better meet the individual needs and lifestyles of our recipients and is the result of persistent efforts to improve hearing outcomes.”
Newly approved products include:
Kanso 2 Sound Processor
The Kanso 2 Sound Processor is reportedly the world’s smallest1 off-the-ear cochlear implant sound processor, and it is the first and only off-the-ear cochlear implant sound processor to offer direct streaming from compatible Apple® or Android™ devices,* according to Cochlear. It is also compatible with the Nucleus Smart App, enabling control of device settings, hearing functions, and information.*

The Kanso 2 Sound Processor features new charging options, including the sound processor’s built-in rechargeable battery2; all-in-one Home Charger, allowing charging, drying, and storing of the sound processor at the same time; and a small, optional portable charger for on-the-go use. The Kanso 2 Sound Processor also features “the highest possible water resistance rating† for any off-the-ear cochlear implant sound processor, giving users the freedom to live an active lifestyle.”
The Kanso 2 Sound Processor features “a simple, durable all-in-one design that makes it easy to use.” Button-free control with an automatic on/off function helps to simplify the experience particularly for children or people with poor dexterity. To help users hear more of what they want to listen to, the Kanso 2 Sound Processor includes Cochlear’s proven hearing performance technologies3-6 of dual microphones, ForwardFocus,¥ and SmartSound® IQ with SCAN**.

At commercial availability, the Kanso 2 Sound Processor is compatible with Cochlear N24, CI24RE, CI500, Profile,® and Profile Plus Series Implants.
Nucleus 7 Sound Processor for Nucleus 22 Implant recipients
The Nucleus 7 Sound Processor is now compatible with a Nucleus 22 Implant. This means that Nucleus 22 Implant recipients can now upgrade to Cochlear’s latest behind-the-ear sound processor, and, “for the first time, benefit from the smallest and lightest behind-the-ear sound processor1 with direct smartphone connectivity and streaming.”
The Nucleus 22 Implant was Cochlear’s first commercial implant, first implanted in 1982 in Australia; it is reportedly “the first FDA-approved cochlear implant in the United States,” obtaining approval in 1985. There are more than 17,000 people around the world with a Nucleus 22 Implant, according to the company; this upgrade means that, for the first time, the first people to hear with a cochlear implant, almost 40 years ago, can now access direct smartphone connectivity.

“The experience and feedback from our hearing implant recipients during the pandemic reminds us that staying connected has never been more important,” said Patricia Trautwein, MA, AuD, Vice President, Product Management & Marketing, Cochlear Americas. “All our recipients, especially the pioneers who trusted in cochlear implant technology first, now will have access to smartphone connectivity in the smallest and lightest devices they’ve ever experienced. We work to deliver innovative new products, so our recipients can have a lifetime of hearing and features that enrich every moment they wish to be part of.”
Custom Sound Pro fitting software
Cochlear has released Custom Sound Pro fitting software to support clinicians programming Cochlear Nucleus Implant sound processors. The software harnesses almost 40 years of experience and input from thousands of clinicians worldwide7.
The Custom Sound Pro fitting software keeps the patient at the center of care with a new patient dashboard and goal-setting feature, helping to “promote engagement and facilitate more effective tracking of progress between appointments8.” With a new layout and increased patient on-air time during fitting, the software is designed to help enhance the programming experience for clinicians and their patients, according to Cochlear.
The new products will be commercially available in the US and Canada later this year. For more information on these products, visit: Cochlear.us/Kanso2. For Cochlear recipients interested in accessing new products, visit: Cochlear.us/Kanso2Upgrade.
References
- Cochlear Ltd. D1190805 Sound Processor Size Comparison. 2020; March. Data on file.
- Cochlear Ltd. D1710313 CP1150 Battery Life Coverage Technical Report. 2020; Mar. Data on file.
- Mauger SJ, Warren CD, Knight MR, Goorevich M, Nel E. Clinical evaluation of the Nucleus 6 cochlear implant system: Performance improvements with SmartSound iQ. International Journey of Audiology.2014;53(8):564-576. [Sponsored by Cochlear].
- Mauger SJ, Jones M, Nel E, Del Dot J. Clinical outcomes with the Kanso off-the-ear cochlear implant sound processor. International Journal of Audiology. 2017;56(4):267-276.
- Wolfe J, Neumann S, Marsh M, et al. Benefits of adaptive signal processing in a commercially available cochlear implant sound processor. Otology & Neurotolology. 2015;36(7):1181-1190.
- Cochlear Limited. D1660797. CP1150 Sound Processor Interim Clinical Investigation Report. January 2020
- Cochlear Limited. D1619303 Software History Timeline. Data on file.
- Dillon H, James A, Ginis J. Client Oriented Scale of Improvement (COSI) and its relationship to several other measures of benefit and satisfaction provided by hearing aids. Journal of the American Academy of Audiology.1997;8:27-43.
* For a full list of smartphone and app compatible devices, visit: www.cochlear.com/compatibility.
** SNR-NR, WNR, and SCAN are FDA approved for use with any recipient ages 6 years and older, who is able to: 1) Complete objective speech perception testing in quiet and in noise in order to determine and document performance; and 2) Report a preference for different program settings.
† The Kanso 2 Sound Processor is dust and water resistant to level of IP68 of the International Standard IEC60529. The Kanso 2 Sound Processor with Aqua+ is dust and water resistant to level of IP68 of the International Standard IEC60529. Aqua+ can be continuously submerged under water to a depth of up to 3 meters for up to 2 hours.
¥ ForwardFocus is a clinician-enabled, user-controlled feature within Custom Sound Pro Fitting Software.
Apple, the Apple logo, FaceTime, Made for iPad logo, Made for iPhone logo, Made for iPod logo, iPhone, iPad Pro, iPad Air, iPad mini, iPad, and iPod touch are trademarks of Apple Inc, registered in the US and other countries. App Store is a service mark of Apple Inc, registered in the US and other countries.
Android is a trademark of Google LLC. The Android robot is reproduced or modified from work created and shared by Google and used according to terms described in the Creative Commons 3.0 Attribution License.
Source: Cochlear
Images: Cochlear