Audiology & Neuroscience | July 2018 Hearing Review
Simple screen methods don’t always come with simple routes for patient counseling
While administering a screening test for dementia is relatively simple, the process and results may trigger significant emotions and anxiety within the patient. The patient may feel confused, vulnerable, and scared. This article addresses these issues, and how to manage and minimize negative thoughts and feelings associated with dementia screenings. Further, this article offers how to use dementia screening processes and outcomes to do what is truly in the best interest of the patient—not just with regard to hearing and listening, but globally, too.
As evidence emerges regarding the negative consequences of untreated hearing loss, the scope of practice for audiologists, and perhaps licensed dispensers, may need to evolve to better meet the demands of the patient. For instance, although the first 100 years of audiology were about hearing,1 the next 100 years will likely be about listening—since listening is where hearing meets the brain.2 In other words, with improved diagnostics, amplification, and aural rehabilitation, the focus is shifting from simply hearing, to the ability to effectively and efficiently communicate. Difficulty communicating is a hallmark of both hearing loss and dementia. Therefore, it is important to evaluate each in appropriate candidates, as either may masquerade as the other!
In this article, we will review the rationale and importance of dementia screenings as indicators of dementia and/or other cognitive problems, and we will suggest a specific screening protocol (endorsed by the Gerontological Society of America) which can be easily and quickly adapted into a clinical office visit, as part of the intake, counseling, or expectations discussion.
The Status Quo
Livingston et al3 reported in The Lancet that “dementia is the greatest global challenge for health and social care in the 21st century.” Currently, across the global, some 50 million people above age 65 years are impacted by dementia, and that number is expected to triple by 2050. Their report states the cost of caring for those with dementia in 2015 was approximately $820 billion, and 85% of those dollars were related to family and social costs, not medical care. Importantly, their report states that hearing loss is often a modifiable risk factor, indicating that if hearing loss is detected and managed sooner, rather than later, the impact of hearing loss on dementia may be (for many) significantly lessened. They state multiple interventions are useful, including pharmacological, psychological, environmental, and social interventions. Livingston et al3 state:
“If these interventions are implemented, people with dementia will have their cognition optimised and they will be less likely to be agitated, depressed, or have troublesome psychotic symptoms…” 3
They reported nine modifiable risk factors for dementia:
1) Diabetes;
2) Social isolation;
3) Hearing loss;
4) Smoking;
5) Obesity;
6) Physical inactivity;
7) Less education;
8) Depression, and
9) Hypertension.
Of those, hearing loss has the largest population attributable fraction (PAF), which was determined to be 9%. Therefore, one can conclude that hearing loss has a significant impact on the cognitive, emotional, and psychological status, and that the earlier people are identified and treated, the better the probable outcome.
Ferguson and colleagues4 concluded in a 2017 Cochrane Review paper that well-fitted hearing aids are effective with regard to improving general health and a hearing-specific, health-related quality of life (QOL). Therefore, for appropriate candidates, it may be useful to evaluate cognitive, emotional, and psychological status via screenings to establish a baseline from which one can re-evaluate later, and to identify who to refer for further diagnostic evaluations with regard to cognitive, emotional, and psychological status.
Beck et al5 reported that sensory perception (ie, hearing loss) impacts cognitive, emotional, and psychological status. Indeed, attenuated, distorted, or absent auditory (or other) sensory perceptions can negatively impact cognitive, emotional, psychological, neurologic, and mental health, and may decrease QOL.
Lin et al6 reported that “hearing loss is independently associated with accelerated cognitive decline and incident cognitive impairment in community dwelling older adults…” Others have reported multiple negative associations between hearing loss and depression, anxiety, societal withdrawal, and decreased QOL.7,8
Beck and Clark9 stated, “Audition matters more as cognition declines and cognition matters more as audition declines” to underscore the co-dependence and interaction of top-down (ie, cognitive) functions with bottom-up (ie, sensory) processes.
At first glance, it seems routine and simple: administer a dementia screening test, score it, and interpret it—done deal! But inevitably, any assessment is also an intervention. Specifically, in this case, a dementia screening may result in patients feeling more vulnerable and scared.
In a previous publication,5 the authors reviewed ways for the hearing care professional (HCP) to explain the rationale for cognitive screening and for minimizing patients’ initial adverse reactions to dementia screenings. Here, we further this analysis and examine some psychological correlates of dementia which may become activated by a screening. We will address ways for the HCP to minimize psychological harm caused by the screening, and ways to utilize the context of the screening test to help patients construct the next phase of their lives.
Psychological Issues Associated with Dementia Screenings
It is important to consider the psychological context of being screened for dementia. Quite often, people with possible dementia are besieged by an endless array of health-related losses, many of which may have been traumatic. Of note, emotional insults and losses rarely exist in isolation. Therefore, actual or suspected loss of cognitive functioning may trigger or reinforce previous negative reactions. Stated more precisely, a cognitive screening may function as a post-traumatic trigger.
While many of the behaviors associated with age-related hearing loss are comparable to those associated with dementia, age-related hearing loss is more precisely and easily measured, whereas dementia is multifaceted, and identifying people at risk is more ambiguous.
There is considerable variability in how dementia manifests and the speed at which it progresses. The impact dementia has on relationships with family and friends, as well as one’s daily life and plans for the future, is almost unpredictable; impaired memory comes and goes in an unpredictable manner and people may experience dementia as an “ambiguous loss” (ie, a loss that occurs without closure or understanding). The same ambiguity delays the grieving process and may result in additional problems, such as unresolved grief.10 To make it worse, this same emotional dynamic becomes more ominous for many patients who experience health-related losses as being one step closer to death. Dementia may cause overall emotional dysregulation, causing indiscriminate emotional outbursts even at the slightest provocation (Table 1).
Minimizing Iatrogenic Effects of Cognitive Screenings
How can HCPs effectively navigate this multi-layered, precarious psychological terrain associated with the potentially negative impact—which may be implied simply by suggesting or administering a dementia screening? Step one is often as simple as asking the patient to self-assess regarding how their memory is functioning. Are they noticing any changes which might cause them concern?
Atul Gawande, MD,11 referenced a strategy palliative care physicians use when giving patients bad news:
Ask, Tell, Ask.
1) They ask patients what information they want to hear.
2) They tell patients that information.
3) They ask what they understood.11
After administering a dementia screening, the HCP may ask a patient, “What would you like to know about the results?” Or, “Would you like to discuss how the results will help you?”
The HCP should not assume the patient wants to hear any or all of the information; hence Dr Gawande’s dictum, “Ask the patient.” The act of patients informing their HCP as to which information they do and not want to hear is an act of empowerment, which may be in marked contrast to their feelings of disempowerment with respect to dementia, hearing loss, and perhaps a host of other health-related losses.
After informing the patient of the result, it is important to ensure their adequate understanding in a way other than asking, “Did you understand what I said?” Patients are likely to react defensively to that approach and/or may pretend to understand. Instead, the HCP should say something like “I just want to make sure I was clear. Let’s go over what I said, as it was a mouthful.”
Of course, any time a positive test result occurs, the HCP needs to be prepared to refer the patient to a psychologist, medical doctor, or other (hopefully local) counselor who can review the screening, gather more data, and create an action plan.
Hearing Care Professionals as Gatekeepers
Age-related hearing loss (ARHL)—which is highly prevalent and for which treatments are available—has recently been identified as a potentially modifiable risk factor. As such, audiologists and HCPs serve as potential gatekeepers who should be poised to recognize changes in memory and communication status which may represent previously undetected warning signs of dementia.
This responsibility requires heightened clinical awareness and a more patient-focused clinical practice.12 For audiologists and all HCPs with established referral networks, dementia screening may be an option when patient behavior (or the concerns of a significant other) indicates that memory impairment may be impacting functional abilities, especially when communicating in effortful listening situations.
Audiologists must be knowledgeable and comfortable with dementia screenings, as well as engaging in conversations regarding memory issues. They should feel comfortable making careful and compassionate decisions, referring to clinicians with expertise in diagnosing and evaluating persons exhibiting signs of dementia, such as memory lapses, behavior changes, social disengagement, and other Neurocognitive Disorders (NCDs).
The Gerontological Society of America Toolkit
The Gerontological Society of America (GSA) recently published a comprehensive toolkit as a resource to assist primary care physicians to detect, diagnose, and manage persons with dementia. The 4-step program is referred to as the “Kickstart-Assess-Evaluate-and Refer” (KAER) program.13
As a complement to the GSA toolkit, Maslow and Fortinsky14 discussed ways in which non-physician care providers can help increase early detection of older persons with dementia. In particular, they emphasized how non-physician healthcare providers can play a role in the diagnostic process and in providing the necessary guidance and support which is very important when individuals are faced with this diagnosis. Maslow and Fortinsky reasoned that, to optimize earlier diagnoses, non-physician healthcare providers can help optimize the systematic detection and referral of persons with cognitive impairment to the appropriate care provider and community resource.
The GSA toolkit includes a list of behavioral indicators suggesting the need for a screening, several reliable and valid dementia screening tools, practical steps regarding discussions about brain health, care planning, and how best to support a person at risk for dementia.15 Behavioral indicators cited include trouble remembering recent events, repeatedly asking the same question, an inability to follow directions easily, and increased confusion over previously routine activities. The GSA toolkit emphasizes the importance of ruling out “non-dementia factors” which may impair cognitive function, such as polypharmacy, depression, or vitamin deficiencies. Untreated hearing loss is not mentioned in these “non-dementia factors,” but clearly may be a factor, especially when administering the cognitive screening tests which are orally administered and depend, to a great extent, on the patient’s ability to hear and listen.
The NIA and Alzheimer’s Association Workgroups identified the Mini-Cog16 as the test of choice, and the GSA has recommended this test as a cognitive screen. The GSA also included two additional cognitive tests in the toolkit, which were recommended by the Alzheimer’s Association Workgroup: the General Practitioner Assessment of Cognition (GPCOG),17and the Memory Impairment Screen (MIS).18 Each screening test is free of charge—unlike the Mini-Mental State Examination (MMSE) or Folstein test19—requires less than 5-10 minutes to administer and score, and can be administered by nurse practitioners or physician assistants. In addition to being quick, easy to administer, and easily accessible, these tests are reliable and valid. The Mini-Cog and the GPCOG are available for free and each has acceptable psychometrics.
In addition to test considerations, variables influencing choice of screening instrument include overall goals, workflow issues, and clinician style. Of note, optimal detection is achieved when screening is combined with inquiry into memory loss as reported by the patient and the informant. Before embarking on case finding/screening activities, audiologists should have established referral networks, consisting of multi-disciplinary professionals (physicians, psychologists, geriatricians, etc) who can help persons with hearing loss and their communication partners learn more about dementia and find needed help in their communities.14
The Mini-Cog: Recommended Cognitive Screening Test
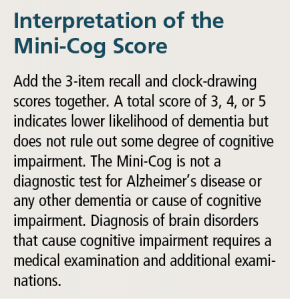
A quick and effective screening tool, the Mini-Cog16 does not require specialized training to administer and is sensitive and specific. Scores are reportedly not impacted by education level, socioeconomic status, ethnicity, or language biases. The Mini-Cog combines two simple cognitive tasks: a three-item delayed- word recall test with a clock-drawing test which serves as an informative distractor and taps visual-spatial abilities. The word-registration part of the test theoretically assures that the words to be used in the recall task have been “registered.” Tables 2-4 provide a description of the process involved in administering the Mini-Cog (also see: https://www.alz.org/documents_custom/minicog.pdf).
Recommendations
In light of emerging evidence associating hearing loss to dementia, hearing loss has been recognized as a modifiable risk factor for dementia. Communication and social connections are critical to brain health, which is why hearing status matters and audiologists and other HCPs have a role to play with regard to screening patients for dementia.
The GSA toolkit provides invaluable tips for kick-starting conversations about raising the awareness and importance of brain health. Also, having a discussion with the patient (and their significant others) about steps to take to protect cognitive health are important. These discussions directly relate to the potential for HCPs to play a more significant role in dementia screening and referral.
Hearing health interventions are designed to optimize communication so people can remain socially, intellectually, and physically active. Recognition of hearing loss as a risk factor for dementia and other negative cognitive consequences remains a potentially new and challenging area of practice, and there is still much work left to be done. We believe audiologists and HCPs have a duty to educate people about the link between better communication through improved hearing and brain health. By improving hearing and optimizing communication, it follows that interventions directed at optimizing communication and social engagement could lessen the probability that hearing loss will “cascade” into cognitive decline.
Douglas L. Beck, AuD, is Executive Director of Academic Sciences at Oticon Inc, Somerset, NJ , is an Adjunct Clinical Professor of Communication Disorders and Sciences at State University of New York, Buffalo, and also serves as Senior Editor of Clinical Research for the Hearing Review’s Inside the Research column. Barbara E. Weinstein, PhD, is Professor & Founding Executive Officer of the Doctor of Audiology Program at City University of New York. Michael Harvey, PhD, ABPP, is a clinical psychologist who specializes in issues related to hearing healthcare and also has a psychology practice in Framingham, Mass.
Citation for this article: Beck DL, Weinstein BR, Harvey M. Dementia screening: A role for audiologists. Hearing Review. 2018;25(7):36-39.
Correspondence can be addressed to Dr Beck at: [email protected]
References
-
Beck DL. Cognition, audition, and amplification. Paper presented at: University of New Zealand at Auckland; Podium Presentation; 2018.
-
Beck DL, Flexer C. Listening is where hearing meets brain in children and adults. Hearing Review. 2011;18(2):30-35. Available at: https://hearingreview.com/2011/10/listening-is-where-hearing-meets-brain-in-children-and-adults-2
-
Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. The Lancet. 2017;39(10113):2673-2734.
-
Ferguson MA, Kitterick PT, Chong L, Edmondson-Jones M, Barker F, Hoare DJ. Hearing aids for mild to moderate hearing loss in adults. Cochrane Database of Systematic Reviews. 2017; 9(CD012023). Available at: http://www.cochrane.org/CD012023/ENT_hearing-aids-mild-moderate-hearing-loss-adults
-
Beck DL, Weinstein BE, Harvey MA. Issues in cognitive screenings by audiologists. Hearing Review. 2016;23(2):36-39. https://hearingreview.com/2016/01/issues-cognitive-screenings-audiologists
-
Lin FR, Yaffe K, Xia J, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173(4):293-299.
-
National Council on Aging. The Consequences of Untreated Hearing Loss in Older Adults. Arlington, Va: NCOA;1999. Available at: https://www.ncoa.org/resources/the-consequences-of-untreated-hearing-loss-in-older-adults/
-
Shield B. Evaluation of the social and economic costs of hearing impairment. A report for Hear-It. October 2006. Available at: https://www.hear-it.org/sites/default/files/multimedia/documents/Hear_It_Report_October_2006.pdf
-
Beck DL, Clark JL. Audition matters more as cognition declines: Cognition matters more as audition declines. March 20, 2009. Available at: https://www.audiology.org/news/audition-matters-more-cognition-declines-cognition-matters-more-audition-declines
-
Boss P. Ambiguous Loss: Learning to Live with Unresolved Grief. Cambridge, Mass: Harvard University Press; 2000.
-
Gawande A. Being Mortal: Medicine and What Matters in the End. New York, NY: Picador/Macmillan Publishers;2017:207.
-
Brayne C, Fox C, Boustani M. Dementia screening in primary care: Is it time? JAMA. 2007;298(20):2409-2411.
-
Gerontological Society of America. Cognitive Impairment Detection and Earlier Diagnosis; KAER Toolkit: 4-Step Process to Detecting Cognitive Impairment and Earlier Diagnosis of Dementia. July 17, 2017. Available at: https://www.geron.org/programs-services/alliances-and-multi-stakeholder-collaborations/cognitive-impairment-detection-and-earlier-diagnosis
-
Maslow K, Fortinsky, RH. Nonphysician care providers can help to increase detection of cognitive impairment and encourage diagnostic evaluation for dementia in community and residential care settings. Gerontologist.2018;58[Supp1]:S20-S31.
-
Graham J. New toolkits help physicans detect, diagnose, and manage dementia. JAMA. 2017;318(14):1310-1312.
-
Borson S, Scanlan JM, Chen P, Ganguli M. The Mini-Cog as a screen for dementia: Validation in a population-based sample. J Am Ger Soc. 2003;51(10):1451-1454.
-
Brodaty H, Pond D, Kemp NM, et al. The GPCOG: A new screening test for dementia designed for general practice. J Am Geriatrics Soc. 2002;50(3):530-534.
-
Buschke H, Kuslansky G, Katz M, et al. Screening for dementia with the Memory Impairment Screen. Neurology. 1999;52(2):231.
-
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res.1975;12(3):189-198.
MORE ON “AUDIOLOGY & NEUROSCIENCE” from this special July 2018 edition of The Hearing Review:
Clinical Speech Audiometry in the Age of the AERP, by James Jerger, PhD.
Cortical Neuroplasticity in Hearing Loss: Why It Matters in Clinical Decision-Making for Children and Adults, by Anu Sharma, PhD, and Hannah Glick, AuD.
Effects of Amplification on Cortical Electrophysiological Function, by Sridhar Krishnamurti, PhD, and Larry Wise, AuD.
Cochlear implants: Considerations Regarding the Relationship between Cognitive Load Management and Outcome, by Edward Overstreet, PhD, and Michel Hoen, PhD.
Dementia Screening: A Role for Audiologists, by Douglas L. Beck, AuD, Barbara R. Weinstein, PhD, and Michael Harvey, PhD, ABPP.