Research | February 2015 Hearing Review
How smartphone apps might help empower more patients to seek amplification.
A self-administered hearing test application could funnel self-motivated patients into the healthcare system who need hearing healthcare services and are self-primed to succeed with amplification technology.
Hearing loss is highly prevalent in older adults. Prevalence estimates indicate that hearing loss, defined as auditory thresholds >25 dB, affects 15% of adults between ages 51 and 60, 37% of adults ages 61-70 years, 60% of adults ages 71-80 years, and more than 80% of adults 85 years and older.1-3 Statistics further indicate that men experience greater hearing loss and earlier onset compared with women.4

Amyn M. Amlani, PhD, is an associate professor of audiology in the Department of Speech and Hearing Sciences at the University of North Texas, Denton, Tex.
The consequences associated with untreated hearing loss result in a myriad of psychological (eg, reduced self-esteem, anger, depression, increased anxiety) and psychosocial (eg, social withdrawal, socially inappropriate behavior or responses, lack of concentration) behaviors, in addition to reduced physical well-being and cognitive decline.5-6 Professionally, the challenge has been, and continues to be, twofold:
1) Increase public awareness about the potential ramifications that stem from untreated hearing loss and provide treatment options, and
2) Reduce the stigma associated with hearing loss and encourage earlier intervention.
Non-compliance to provider recommendations and adopting amplification by listeners is related to the individual’s perception that their hearing loss is not sufficient to warrant assistance.7 Preliminary data from our laboratory and supported by research8 suggest that terms such as “mild” and “hearing loss” are negatively impacting consumer behavior toward hearing healthcare services and technology.
?The Role and Effectiveness of Hearing Screenings
Hearing screenings are a common approach used by professionals to increase public awareness and promote early intervention. Hearing screenings performed on adults, unfortunately, have less than 20% provider compliance for further evaluation and remediation.9-10 Thus, there is a need to explore novel strategies that could potentially enhance screenings as a feasible component of routine hearing healthcare.
Recently, it has been suggested that smartphone-based applications improve patient compliance to healthcare treatment in adults.11 This increase in compliance to treatment stems from the smartphone-based application providing:
1) Patient information about their treatment and condition;
2) A means by which patients can access their provider and vice-versa, allowing for the patient to ask questions about their treatment; and
3) The ability for the end user to store and reference previous screening results.
Together, these attributes have shown an increase in perceived self-efficacy, or a heightened increase in an individual’s confidence to perform a specific behavior.12 Research indicates that perceived self-efficacy increases the likelihood of compliance to provider recommendations in healthcare patients.13
This article reports on results from a study completed in our laboratory comparing the benefits of older adults complying with provider recommendations from a hearing screening when the initial information is provided face-to-face versus via a self-administered smart-phone-based hearing test application.
Methods
Participants. We recruited two groups of listeners, each having 104 participants. Listeners in Group 1 (57 females [mean age = 67.5 years; SD = 6.8]; 47 males [mean age = 64.5 years; SD = 7.3]) had their air-conduction hearing sensitivity assessed using traditional audiometric means. Listeners in Group 2 (44 females [mean age = 65.1; SD = 5.8]; 60 males [mean age = 64.2; SD = 9.2]) had their air-conduction hearing sensitivity assessed using a smartphone-based hearing test application. We recruited participants from senior centers and active adult retirement communities in the Dallas-Fort Worth (DFW) metropolitan area, based on the following criteria: 1) age between 50 and 90 years; 2) perceived bilateral hearing loss; and 3) no previous experience with amplification.
Traditional air-conduction screening. We obtained responses to air-conduction pure-tone stimuli for participants in Group 1 using a calibrated screening audiometer (Grason- Stadler [GSI] Model 18) coupled to TDH 39 supra-aural headphones. After an otoscopic inspection of both ears, participants were seated in a chair with their back angled 90° to the experimenter. Participants responded by raising their hand when they heard an auditory stimulus (ie, pulsed pure-tone) played through the headphones in a given ear. The right ear was always tested first. We presented pulsed pure-tones at 30 dB for 500 Hz, and 20 dB at the frequencies of 1000, 2000, and 4000 Hz.
At the conclusion of the test, we counseled each participant using a written script that indicated, when appropriate, the need for further diagnostic testing. We informed participants that the additional diagnostic testing would be performed at the University of North Texas facility free of charge. The experimenters made no mention during the counseling process of the need for amplification. In the event that the participant asked about amplification, they were told that further testing was needed to determine candidacy.

Figure 1. Screen shot of the testing setup page from the EarTrumpet hearing test application. See text for details.
Self-administered smartphone-based hearing test application. For participants in Group 2, air-conduction thresholds were tested by means of a smartphone-based hearing suite application called EarTrumpet (Praxis BioSciences, LLC), downloaded from iTunes and installed on an Apple iPad (2nd Generation). The hearing suite consists of two modules: a hearing enhancer (ie, sound amplifier) and hearing test. In this study, only the latter module was utilized. Specifically, pure-tone signals generated by the application were delivered to the participant’s ears using the accompanying Apple hardwired in-ear head-phones, which coupled to the iPad audio jack by means of a standard 3.5-mm connector.
The self-administered hearing test module allows for three test types and the option to select a headphone transducer. Test types include Basic (ie, thresholds obtained at 500, 1000, 4000, 8000 Hz), Comprehensive (ie, thresholds obtained at audiometric octave frequencies between 125 Hz and 8000 Hz), and Custom (ie, allows for thresholds to be selected for testing). For this study, we opted for the Basic test type (Figure 1), as we felt it best represented a hearing screening paradigm. EarTrumpet also allows for the selection of a headphone transducer, with application pre-calibrated for the accompanying Apple in-ear headphones.
During testing, we left a printed instruction set on the table guiding the participant through the hearing test process. First, each participant coupled the headphone transducer to the respective ear. (We did not perform an otoscopic inspection on this group prior to their participation.) Second, the participant calibrated the volume level of the headphone’s output to a predetermined level, depicted on the iPad screen by means of a sliding bar and a green check mark (Figure 1). Third, the participant initiated the hearing test by pressing a button box labeled “start,” which then defaulted to the application running a 5-second analysis of the levels in the environment. Once the environment was considered satisfactory for testing, the application screen defaulted to a new screen that displayed a button box instructing the participant to press the button when they heard a series of three pulsed pure-tones. Thresholds at each frequency were determined in 5 dB intervals and after the third ascending reversal. The right ear was the default ear at the beginning of the hearing test.

Figure 2. Screen shot of air-conduction thresholds results taken from a hypothetical participant using the EarTrumpet hearing test application.
After air-conduction thresholds had been obtained on both ears, the application graphed the results on an audiogram (Figure 2). Then, participants were instructed to press the “details” tab, which provided an explanation of the audiometric findings for each ear and provided potential rationales for the results in the comments section (Figure 3).
After reading the findings, we asked each participant to either store their data on the iPad or exit the application without storing the data. Participants who elected to store their data were instructed to maintain their privacy by entering the first initial of their given name, the last four digits of their social security number, and the last letter of their family name. We also made available flyers with our telephone number and e-mail address, informing participants of the opportunity to undergo diagnostic testing at our facility free of charge.

Figure 3. Screen shot explaining the audiometric findings and potential rationales from a hypothetical participant using the EarTrumpet hearing test application.
As with Group 1, no mention was made by the experimenters of the need for amplification. In the event that the participant asked about amplification, they, too, were told that further testing was needed to determine candidacy.
Self-Efficacy Scale. At the conclusion of the hearing test component, participants from both groups completed the General Self-Efficacy (GSE) scale.14 The GSE scale consists of 10 items that reflect the respondent’s beliefs regarding their ability to perform difficult tasks and cope with adversity. This paper and pencil test was designed for the general adult popula- tion, beginning with adolescents aged 12 years and older. Respondents were asked to answer each question using a 4-point scale, ranging from: 1) Not at all true, to 4) Exactly true. The final composite score for a given respondent was calculated by summing the responses, with the lowest score being 10 and the highest score being 40. Scores closer to 40 represent a high perception of self-efficacy. In this study, we hypothesize that respondents with higher scores are more likely to comply with referrals for additional evaluation and remediation.
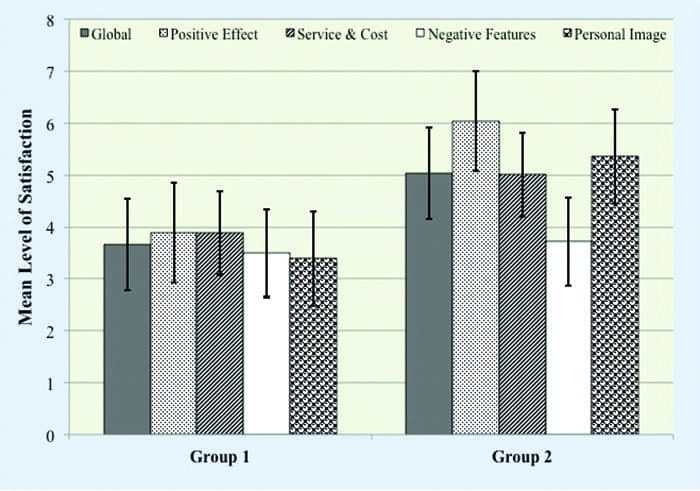
Satisfaction with Amplification Scale. We administered the Satisfaction with Amplification in Daily Life (SADL)15 scale to those participants, in both groups, who complied with additional evaluation and remediation recommendations 3 months after hearing aid use. The SADL is a 15-item self-report measure that assesses the multidimensional nature of satisfaction related to aspects of hearing aid use. The participants’ task was to rate their level of satisfaction ranging from A (ie, not at all) to G (ie, tremendously). This scale provides a global score indicating overall satisfaction, as well as four subscale scores profiling satisfaction in the areas of: 1) Positive Effect (ie, improved psychoacoustic and psychological functioning); 2) Service and Cost (ie, value for money, confidence in provider); 3) Negative Features (ie, undesirable effects of hearing aids, including background noise and feedback); and 4) Personal Image (ie, appearance, stigma).
Procedures. Prior to undertaking this study, octave-band sound-level measurements were made in each conference room during normal business hours. Each room contained between 3 and 5 chairs and a rectangular table draped with a tablecloth.
All participants enrolled in this study were seen within 3 months of their hearing test. During data collection, each participant was seated individually in a small conference room located within the senior center or retirement community clubhouse. Participants completed the hearing testing component first, then completed the self-efficacy (GSE) questionnaire.
Participants who contacted us for further diagnostic testing were provided, free of charge, a comprehensive audiological assessment (ie, air- and bone-conduction testing, immittance measures, otoacoustic emissions, word-recognition testing). Participants, in both groups, who responded favorably to recommendations, were able to purchase amplification and were provided the same standard services (ie, real-ear measures, electroacoustic testing, speech-in-noise testing, counseling, rehabilitation strategies). After 3 months of hearing aid use, we measured each participant’s satisfaction.
Results and Discussion
Hearing screening compliance. Results for Group 1 revealed that 5 participants out of 104 passed the traditional hearing screening. Of the 99 participants who failed the hearing screening, only 15 listeners (ie, 15.2%) complied with experimenter recommendations for additional evaluation and potential remediation. In addition, one participant who passed the hearing screening scheduled an appointment for additional follow-up concerning his difficulty to hear speech against competing noise.

Figure 4. Audiometric thresholds and ±1 standard deviation for Group 2 participants’ right and left ears obtained at 500, 1000, 4000, and 8000 Hz from the EarTrumpet smartphone-based hearing test application.
Similarly, only 2 out of 104 participants in Group 2 exhibited normal-hearing sensitivity thresholds using the self-administered smartphone-based hearing test application, while threshold results for the remaining 102 respondents indicated decreased air-conduction thresholds. Figure 4 displays mean audiometric thresholds and ±1 standard deviation for Group 2 participants’ right and left ears who demonstrated potential hearing loss. Of the 102 participants whose results indicated a decrease in auditory sensitivity, 31 listeners (ie, 30.4%) e-mailed their results and requested a follow-up diagnostic appointment. Together, these data further suggest that the odds of referral double (ie, 31 participants in Group 2 divided by 15 participants in Group 1) using a self-administered hearing test application compared to the traditional hearing screening.
Accuracy of the Self-Administered Smartphone-Based Hearing Test Application
We measured the accuracy of the self-administered smartphone-based hearing test application to pure-tone clinical audiometry by calculating the mean difference across the frequencies of 500, 1000, 4000, and 8000 Hz for the 31 listeners in Group 2. We defined mean difference as the self-administered hearing test threshold minus the pure-tone audiometric threshold at a given frequency across participants. A positive mean difference indicated that the self-administered smartphone-based hearing test application overestimated threshold compared to the pure-tone audiometric threshold at that frequency. A negative mean difference indicated that the pure-tone audiometric threshold overestimated threshold compared to the self-administered smartphone-based hearing test application at that frequency.
Results suggest that the average mean difference was within ±5 dB at all frequencies (p > .05). The large standard deviations, however, suggest large inter-subject variability, which might have been influenced by factors such as ambient noise in the test environment, poor placement of the earbuds in the ear canal, and differences in the frequency response between the calibrated headphones used in clinical audiology and the Apple in-ear headphones.
Self-Efficacy Scale. Figure 5 shows results from the GSE scale by group. Overall, Group 1 reported a mean self-efficacy of 17.85 (SD = 4.90), while Group 2 reported a mean self-efficacy of 27.69 (SD = 4.48). A Wilcoxon Mann-Whitney test comparing mean self-efficacy between groups was statistically significant (Mann-Whitney U; Z = 2.43, p < .05).
Next, we dichotomized the data into those individuals who complied with recommendations versus those who did not (ie, non-compliers) for each group. Within each group, results revealed that mean self-efficacy scores were similar (p < .05) between those participants who complied and those who did not. Between groups, findings revealed a significantly larger mean self-efficacy score for participants in Group 2 who complied with smartphone-application recommendations (Mann-Whitney U; Z = 4.91, p < .001) compared to participants in Group 1 who also complied with recommendations during a traditional hearing screening. No statistical significance (p > .05) was found between non-compliers in Groups 1 and 2.
The findings point toward an increase in self-efficacy promoted by the smartphone-based hearing test application over the traditional hearing screening approach. However, this claim is tenuous, at best, given that we did not quantify self-efficacy before and after each participant’s hearing test.
Hearing Aid Adoption. A secondary component of this study was to assess the degree to which self-efficacy increased hearing aid adoption. Recall, 15 participants in Group 1 (ie, traditional hearing screenings) made self-referral appointments for additional diagnostic testing. Of these 15 participants, 11 had sufficient hearing loss that required amplification, but only 4 participants underwent and completed a trial period with amplification.
For Group 2 (ie, self-administered smart-phone-based hearing test application), 31 participants requested an appointment for additional diagnostic testing. Of these 31 participants, 21 had sufficient hearing loss that required amplification and 15 participants underwent and completed a trial period with amplification. The data indicate a statistically significant (p < .05) advantage in conversion (ie, closure) rate for the smartphone-based hearing test application (ie, 71.4% or 15/21) compared to the traditional hearing screening (ie, 36.4% or 4/11).
Satisfaction. For the 19 participants (Group 1 = 4; Group 2 = 15) who adopted amplification, we assessed their multidimen- sional perception of satisfaction related to hearing aid use using the SADL. We collected this data 3 months post fitting. A Wilcoxon Mann-Whitney test revealed statistically significant differences between groups in global satisfaction (Mann-Whitney U; Z = 2.11, p < .05) and in the subscales of Positive Effect (Mann-Whitney U; Z = 4.57, p < .001), Service and Cost (Mann-Whitney U; Z = 1.99, p < .05), and Personal Image (Mann-Whitney U; Z = 3.06, p < .001). Interestingly, there was no statistical difference between groups for the Negative Features subscale (p > .05), indicating that participants in both groups perceived similar satisfaction. For the individual questions that constitute the Negative Features subscale, namely questions 2, 7, and 11, responses were similar (p > .05).
The satisfaction data suggest that participants in both groups face similar negative issues (ie, understanding speech in noise, feedback) from the technology, yet participants in Group 2 demonstrated an increased satisfaction with the audiological services and technology they were provided.
Conclusions
The self-administered smartphone-based hearing test application is neither intended to replace standard pure-tone audiometry nor the services provided by the hearing healthcare provider. Instead, the benefit of this technology offers the hearing healthcare community the opportunity to:
1) Raise public awareness related to the consequences of hearing impairment, and
2) Promote early intervention.
The specific self-administered smartphone-based hearing test application used in this study yielded an increase in self-efficacy in Group 2 participants compared to participants in Group 1 who experienced the traditional hearing screening model. This increase in self-efficacy improved the likelihood—by a factor of 2:1—of impaired listeners seeking the services and technologies offered by hearing healthcare providers. For participants who purchased amplification, increased self-efficacy provided by the self-administered smartphone-based hearing test application promoted greater satisfaction with provider services and with the same technology, despite similar perceptual difficulties between groups while listening to speech-in-noise and with feedback. Our findings suggest that providing self-administered hearing testing applications might be one avenue to increasing the historically stagnant growth in this market.
Globally, we conjecture that self-administered hearing test applications could be used as part of a hearing wellness program. This is particularly important given the increasing demand for hearing healthcare services in the coming decades and the unmet supply of professionals entering the profession.16-17 The concept of a self-administered hearing test application could yield, for example, a funnel of self-motivated patients into the healthcare system, who need hearing healthcare services and are self-primed to succeed with amplification technology.
Acknowledgement
The author thanks Timothy A. Russo for his assistance with the hearing screenings.
References
1. Gates GA, Cooper JC Jr, Kannel WB, Miller NJ. Hearing in the elderly: The Framingham cohort, 1983-1985. Part 1. Basic audiometric tests results. Ear Hear. 1990;11(4):257-256.
2. Van Eyken E, Van Camp G, Van Laer L. The complexity of age-related hearing impairment: contributing environmental and genetic factors. Audiol Neurootol. 2007;12(6):345-358.
3. Chien W, Lin FR. Prevalence of hearing aid use among older adults in the United States. Arch Intern Med. 2012;172(3):292-293.
4. Lee FS, Matthews LJ, Dubno JR, Mills JH. Longitudinal study of pure-tone thresholds in older persons. Ear Hear. 2005;26(1):1-11.
5. Ciobra A, Bianchini C, Pelucchi S, Pastore A. The impact of hearing loss on the quality of life of elderly adults. Clin Interventions Aging. 2012;7:159-163.
6. Lin FR, Yaffe K, Xia J, Xue Q, Harrris TB, Purchase-Helzner E, Satterfield S, Ayonayon HN, Ferrucci L, Simonsick EM. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173(4):293-299.
7. Kochkin S. MarkeTrak VIII: The key influencing factors in hearing aid purchase intent. Hearing Review. 2012;19(3):12-25.
8. Alcock C. Trigger happy hearing: Using social triggers to promote regular hearing checks. Audiology Practices. 2014;6(2):8-15.
9. Milstein D, Weinstein BE. Effects of information sharing on follow-up after hearing screening for older adults. J Acad Rehab Audiol. 2002;35:43-58.
10. Thodi C, Parazzini M, Kramer SE, Davis A, Stenfelt S, Janssen T, Smith P, Stephens D, Pronk M, Anteunis L, Schirkonyer V, Grandori F. Adult hearing screening: Follow-up and outcomes 1. Am J Audiol. 2013;22:183-185.
11. Krishna S, Boren S, Balas A. Healthcare via cell phones. Telemedicine and e-Health. April 2009:231.
12. Bandura A. Social cognitive theory: An agentic perspective. Annu Rev Psychol. 2001;52:1-26.
13. Maibach E, Flora JA, Nass C. Changes in self-efficacy and health behavior in response to a minimal contact community health campaign. Health Communication. 1991;3(1):1-15.
14. Schwarzer R, Jerusalem M. Generalized Self-Efficacy scale. In: Weinman J, Wright S, Johnston M, eds. Measures in Health Psychology: A User’s Portfolio. Causal and Control Beliefs. Windsor, UK: NFER-NELSON; 1995:35-37.
15. Cox RM, Alexander GC. Measuring satisfaction with amplification in daily life: The SADL Scale. Ear Hear. 1999;20:306-320.
16. Swanepoel D, Clark JL, Koekemoer D, Hall JW, Krumm M, Ferrari D, McPherson B, Olusanya B, Mars M, Russo I, Barajas J. Telehealth in audiology—the need and potential to reach underserved communities. Int J Audiol. 2010;49:195-202.
17. Windmill IM, Freeman BA. Demand for audiology services: 30-year projections and impact on academic programs. J Am Acad Audiol. 2013;25:407-416.
Correspondence can be addressed to Dr Amlani at: [email protected]
Citation for this article: Amlani.A. Improving Patient Compliance to Hearing Healthcare Services and Treatment through Self-Efficacy and Smartphone Applications. Hearing Review. 2015;21(2):16.