In a report presented July 20 at the Alzheimer’s Association International Conference 2017 (AAIC 2017) in London, The Lancet International Commission on Dementia Prevention, Intervention, and Care said that more than one-third of global dementia cases may be preventable through addressing lifestyle factors that impact an individual’s risk, the AAIC announced. These potentially modifiable risk factors—including hearing loss—have been identified at multiple phases across the lifespan, not just in old age.
The Lancet Commission’s report was simultaneously published in The Lancet and presented at AAIC 2017.
Also at AAIC 2017, the National Institute of Health’s (NIH) National Institute on Aging (NIA) announced inaugural research grants to investigate health disparities in Alzheimer’s disease.
“Today’s findings are extremely hopeful,” said Maria Carrillo, PhD, chief science officer at the Alzheimer’s Association. “At an individual level, many people have the potential to reduce their risk of cognitive decline, and perhaps dementia, through simple, healthful behavior changes. At a public health level, interventions based on this evidence could be extremely powerful in managing the global human and economic costs of Alzheimer’s disease and other dementias.”
The Alzheimer’s Association—a Chicago-based organization that focuses on providing care and support to those affected by Alzheimer’s—has compiled a guide called 10 Ways to Love Your Brain, which includes practical guidance to reduce your dementia risk based on the latest research.
The Lancet International Commission on Dementia Prevention, Intervention, and Care
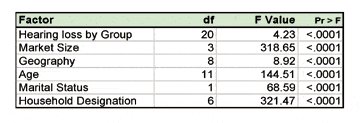
According to the AAIC, The Lancet Commission brings together 24 international experts to consolidate the huge strides that have been made in our knowledge and understanding of dementia risk factors, treatment, and care, and the emerging knowledge as to what we should do to prevent and manage dementia. The Commission conducted a new review and meta-analysis; they extended current models of risk by including hearing loss and social isolation. Incorporating potentially modifiable risk factors from across a lifespan, they proposed a novel life course model of risk, highlighting the opportunity for prevention.
Among their key recommendations are:
- Be ambitious about prevention. Interventions for established risk factors may have the potential to delay or prevent one third of dementias.
- Treat cognitive symptoms. To maximize cognition, people with Alzheimer’s dementia or dementia with Lewy bodies should be offered cholinesterase inhibitors at all stages, or memantine for severe dementia.
- Individualize dementia care. Good dementia care spans medical, social, and supportive care and should be tailored to unique individual and cultural needs, preferences, and priorities.
- Care for family carers. Family carers are at high risk of depression. Effective interventions reduce the risk and treat the symptoms, and should be made available.
- Plan for the future. People with dementia and their families value discussions about the future and important upcoming decisions.
- Manage neuropsychiatric symptoms. Management of the neuropsychiatric symptoms of dementia—including agitation, low mood, or psychosis—is usually psychological, social, and environmental, with drug treatment reserved for more severe symptoms.
- Consider end of life. A third of older people die with dementia, so it is essential that professionals working in end-of-life care consider whether a patient has dementia as they may be unable to make decisions about their care or express their needs and wishes.
Focus on Prevention
The Lancet Commission launched a novel lifespan-based model of dementia risk, showing interventions that may maximize cognition, decrease distressing associated symptoms, reduce crises, and improve quality of life, according to the AAIC announcement. The team estimate the contribution of each of the risk factors to the overall incidence of dementia, at the population level. The combined evidence to date shows that roughly 35% of all cases of dementia are attributable to nine potentially modifiable risk factors. Many of the risk factors occur at particular life stages but some, such as smoking and hypertension, are likely to make a difference at all life stages. The nine modifiable risk factors include:
- Early life – Education to a maximum of age 15
- Mid-life – Hypertension, obesity, hearing loss
- Later life – Depression, diabetes, physical inactivity, smoking, low social contact
Risk factors that are more common account for a higher percentage of population risk. For instance, the authors estimate that 8% of all dementia cases could be associated with poor early school education and 5% could be associated with smoking. While the mechanism linking education, hypertension, diabetes, and smoking to dementia is relatively well understood, the recognition of hearing loss as a potential risk factor is still new, and the research is at an earlier stage, said AAIC. The Hearing Review has published two special editions on emerging research into hearing loss and cognition in the September 2012 and September 2015 issues.
The Commission’s report delivered recommendations for targeted public health strategies that the researchers expect will significantly lower the global burden of Alzheimer’s and other dementias. For example:
- The authors strongly recommend vigorously treating hypertension in middle-aged and older people without dementia to reduce dementia incidence.
- Other recommended interventions include more childhood education, getting regular exercise, maintaining social engagement, stopping smoking, and management of hearing loss, depression, diabetes, and obesity.
The authors stated that, due to lack of data, the study did not include dietary factors, alcohol use, visual impairment, air pollution, and sleep.
“While public health interventions will not prevent, or cure all potentially modifiable dementia, intervention for cardiovascular risk factors, mental health, and hearing may push back the onset of many people for years,” said Professor Gill Livingston, MD, from University College London and lead author of The Lancet Commission. “Even if some of this promise is realized, it could make a huge difference and we have already seen in some populations that dementia is being delayed for years. Dementia prevalence could be halved if its onset were delayed by five years.”
According to the Commission’s report, worldwide dementia prevalence could be reduced by more than 1 million cases with a 10% reduction in the prevalence of seven principal health and lifestyle factors. An intervention that delayed dementia by a year might decrease the number of people living with dementia globally by 9 million in 2050.
“Overall, there is good potential for prevention and, once someone develops dementia, for care to be high-quality, accessible, and give value to an underserved, growing population,” said Lon Schneider, MD, from the University of Southern California and co-author of the Commission. “Effective dementia prevention and care could transform the future for society and vastly improve living and dying for individuals with dementia and their families. Acting now on what we already know can make this difference happen,” said Schneider.
Advancing Health Disparities Research in Alzheimer’s —National Institute on Aging Inaugural Grants
According to the Alzheimer’s Association 2017 Alzheimer’s Disease Facts and Figures, African-Americans are about twice as likely to have Alzheimer’s or other dementias as older whites, and Hispanics are about one and one-half times as likely to have Alzheimer’s or other dementias as older whites. Yet, these populations are underrepresented in Alzheimer’s and dementia research.
According to the AAIC announcement, the NIA has identified a clear need to diversify research cohorts and improve methods and tools for conducting health disparities research related to Alzheimer’s disease and other dementias. Two funding opportunities were created to encourage research that examines disparities in Alzheimer’s disease using diverse cohorts of subjects. At AAIC 2017, NIA will announce the inaugural grant recipients and their projects, and highlight the new information expected to be generated because of these awards.
“Aging research using a framework that incorporate factors at multiple levels needs to be conducted with study populations that have robust demographic diversity,” said Carl V. Hill, PhD, MPH, director of the NIA Office of Special Populations. “When cohorts are diverse, new pathways that link environmental, sociocultural, behavioral, and biological factors can be identified. This is our hope for these research awards.”
According to the funding opportunity announcements, health disparities populations include: Blacks/African-Americans, Hispanics/Latinos, American Indians/Alaskan Natives, Asian-Americans, Native Hawaiians and Other Pacific Islanders, Socioeconomically Disadvantaged Populations, and Rural Populations. Additional populations may include: Disability Populations, and Sex and Gender Minorities.
Source: AAIC
Image: AAIC website





I am resident in the UK. I wonder which ‘avenue’ is better to pursue for hearing aids : National Health Service or Private Practice ?
Thankyou.
Hi Robert , As an Audiologist I must point out that none of these reports on Alzheimer’s prevention can say ‘Treating hearing loss’ can prevent dementia. The only comment these reports can make is there is a correlation between hearing loss and dementia. Does hearing loss cause dementia?, does hearing loss affect the rate of dementia decline ? or does dementia cause hearing loss ? are all areas to be researched in the future. It is quite possible there is a common factor e.g. vascular issues. However, if someone has early dementia and hearing loss it would be important to get them hearing aids to prevent poor communication, frustration and stress. regards