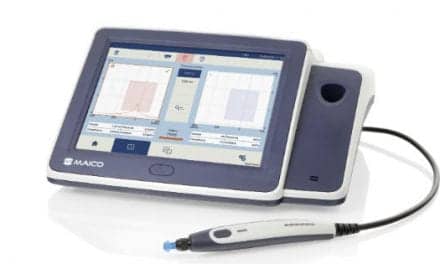
Availability of Neuromod’s tinnitus treatment device, Lenire, has expanded in Spain and is now available in the cities of Malaga, Seville, and Granada through a partnership with medical technology distributor, TRUSBIN.
Lenire’s Spanish launch began in Barcelona through the hearing care center, Teknon Clinic, led by otorhinolaryngologist Jordi Coromina, PhD. This latest expansion brings the number of clinics in Spain providing Lenire to four, according to Neuromod.
The Bimodal Neuromodulation Device
Lenire is a bimodal neuromodulation device designed to provide long-term relief from tinnitus that sustains for at least 12-months in large scale clinical trials.(1,3) In Lenire’s most recent controlled clinical trial, the device was shown to be more effective than sound-alone for those with moderate or worse tinnitus.(3)
The Global Impact of Tinnitus
Tinnitus is a complex brain signaling condition that causes people to perceive sound with no external source. An estimated 15% of the global adult population is impacted by tinnitus, which means more than 7.5 million people in Spain could be living with the condition.(4)
“Barcelona was an ideal location to launch Lenire in due to the city’s rich tradition in the medical field,” says Neuromod Founder and CEO, Ross O’Neill, PhD, MBA. “Neuromod’s partnership with TRUSBIN has allowed us to expand Lenire’s availability to meet the underserved demand for tinnitus care in Spain, while guaranteeing the highest level of patient care in state of the art hearing care clinics.”
Further reading: How Tinnitus Symptoms in Musicians Vary by Age
Neuromod’s Partnership with TRUSBIN
Neuromod’s commercial partner in Spain, TRUSBIN, is a medical device distributor in Spain. TRUSBIN is responsible for identifying leading hearing care clinics, creating partnerships, and training clinics in administering treatment with the device.
“Partnership with Neuromod enables us to bring life-changing tinnitus treatment technology to the millions living with tinnitus in Spain,” says Xavier Caceres, TRUSBIN CEO. “Launching in three leading hearing clinics in Granada, Malaga, and Seville allows us to address the surging demand for clinically proven treatment options for the underserved condition.”
Lenire launched in Europe in 2019 following the success of two large-scale clinical trials, TENT-A1 and TENT-A2. These clinical trials represented the largest ever conducted for a tinnitus treatment device and featured the longest follow-up timeframe.
Clinical Trials of the Tinnitus Treatment Device
The first of Lenire’s clinical trials, TENT-A1, represents one of the largest and longest followed-up clinical trials ever conducted in the tinnitus field and was the cover story for the top-tier peer reviewed scientific journal, Science Translational Medicine, the company says. The trial enrolled 326 participants and 86.2% of compliant participants reported an improvement in their tinnitus severity after a 12-week treatment period.(1) When followed up with 12 months post treatment, 80.1% of compliant participants had sustained improvement.(1)
Results from Lenire’s second large-scale clinical trial, TENT-A2, were published in the prestigious scientific journal, Nature – Scientific Reports. TENT-A2 data demonstrated that modifying stimuli halfway through treatment resulted in a greater clinically significant improvement in tinnitus severity.(2) 95% of compliant patients reported a tinnitus improvement, 91% of whom reported a sustained improvement for a year after the treatment ended.(2)
Lenire Gets FDA De Novo Grant
Lenire’s was recently awarded a De Novo Grant from the U.S. FDA based on the success of the device’s third large-scale clinical trial, TENT-A3. During this controlled clinical trial, 79.4% of the patients had a clinically significant reduction in tinnitus severity and 88.6% responded that they would recommend Lenire.(3) Importantly, Lenire was proven to be more effective than sound-only therapy for 70.5% of patients with moderate and above tinnitus.(3) TENT-A3’s landmark results are set for publication in an independent scientific journal in 2024.
Lenire is a bimodal neuromodulation device that works by delivering mild electrical pulses to the tongue, through an intra-oral component called the “Tonguetip,” combined with auditory stimulation through headphones to drive long-term changes in the brain to treat tinnitus. This dual action stimulus is proven to provide long term relief from tinnitus.(1,2)
Lenire is now available in more than 30 clinics across Europe and the United Kingdom with an additional 70 clinics in the United States of America.
Photo: Neuromod
References:
- Conlon et al., Sci. Transl. Med. 12, eabb2830 (2020)
- Conlon et al., Different bimodal neuromodulation settings reduce tinnitus symptoms in a large randomized trial, Sci Rep, https://www.nature.com/articles/s41598-022-13875-x (2022)
- TENT-A3 clinical trial data in preparation for publication. https://clinicaltrials.gov/ct2/show/NCT05227365 4. https://www.nidcd.nih.gov/health/tinnitus
- R. Biswas et al., Tinnitus prevalence in Europe: a multi-country cross-sectional population study, The Lancet Regional Health (2021), https://doi.org/10.1016/j.lanepe.2021.100250