Current research supports a case for audiologists providing cognitive screenings to meet the needs of certain patients.
By Douglas L. Beck, AuD, F-AAA, CCC-A; Rhee Nesson, AuD, CCC-A; and Laiba Ishtiaq, BA
Despite vast and irrefutable evidence that early diagnosis and treatment of hearing loss leads to the best outcomes, the U.S. Preventive Services Task Force (USPSTF) does not recommend hearing screenings for older Americans. Recent estimates of hearing loss prevalence state 65.3% of adults 71 years and older, 76.7% ages 80-84 years, and 96.2% of adults aged 90 years and older (75% of those aged 90+ years had moderate or greater) have hearing loss.1
Further Reading: What the ACHIEVE Study Tells Us About Hearing Intervention and Cognition: An Interview with Jennifer Deal, PhD
Likewise, the USPSTF does not recommend cognitive screenings. Manly, Jones, Langa, et al estimated that within the U.S. (based on 3,496 older adults with an average age of 76.4 years) 10% had dementia and 22% were classified as having mild cognitive impairment (MCI).2 Others estimate that in the U.S., 1 of 5 adults over age 65 years has MCI and one-third of adults over age 85 have Alzheimer’s disease and related disorders (ADRD).
But just because the USPSTF does not necessarily recommend screenings doesn’t mean they can’t or shouldn’t be performed.
Audiology Scope of Practice
Diagnostic audiometric tests and cognitive screenings are within the scope of practice of audiologists.3-4 As the U.S. population ages and older adults seek social engagement, audiologists will see an increasing number of people with hearing, listening, and cognitive problems.5 For older adults in particular, early detection of sensory deficits and potential cognitive impairment helps facilitate timely medical intervention and social support.6
Kricos noted hearing loss and cognitive problems could mask or parade as each other and may involve multiple challenges including sensory, central, cognitive, psychological, emotional, cognitive, auditory processing, and more.7 Kricos observed, simply testing peripheral hearing is not sufficient as communication problems may involve cognitive and/or auditory processing impairments. With all of this in mind, Kricos stated that we certainly need more information about older adults with cognitive and auditory processing issues; however, “we will not serve them well if we wait for definitive answers about how best to assist them.”
When an audiologist performs a cognitive screening, the result helps determine whether the communication problem is primarily an audiologic issue, or whether a medical referral (based on a positive cognitive screening) is the more prudent recommendation.
For many years our recommendation has been that patients with positive outcomes on their cognitive screenings should be referred to their primary care physician (PCP) or referring physician for further evaluation (diagnostic cognitive testing and treatment are not within the scope of practice of audiologists).
Access to Medical Tests and Healthcare in 2024
Without benefit of diagnostic testing, professional recommendations, guidance, or perspective, people can legally engage in their own diagnostics and treatment without a professional. Over-the-counter (OTC) hearing aids have been FDA approved and available for almost two years. Indeed, people can obtain a personalized Alzheimer’s biomarker assessment without consulting licensed healthcare providers to help them understand the meaning and implications of the results.
Arias, Manchester, and Lah report Quest Diagnostics introduced the first direct-to-consumer (DTC) Alzheimer’s biomarker test.8 The test includes a blood draw and report on the consumer’s amyloid status, without a physician’s order. For years in the United States, the public has been able to obtain private-pay testing for sexually transmitted diseases (STD, aka 10 Test Panel), pregnancy, AIDS/HIV (enzyme-linked immunosorbent assay, ELISA assay), COVID-19, A1C measures, colon cancer screenings (guaiac test), and MRI imaging for tumors, cancers, and cardiac and vascular anomalies. All can be obtained without professional involvement.
However, a lack of support, guidance, context, and meaning from a healthcare professional when receiving test results can be difficult for patients.
Health interventions, such as dementia screenings, offer the potential for people to remain socially, intellectually, and physically active, which is certainly a positive. Beck, Weinstein, and Harvey reported that although performing a screening test for dementia is relatively simple, the protocol itself and the outcome could certainly trigger significant emotions and anxiety for the patient.9 Therefore, they addressed ways for hearing care professionals (HCPs) to manage and minimize negative thoughts and feelings associated with dementia screenings.
They reported communication and social connections are critical to brain health, and therefore HCPs have a role to play regarding screening patients for cognitive issues. Interventions directed at optimizing communication and social engagement could lessen the probability that hearing loss will “cascade” into cognitive decline.5
Who Screens?
Many professionals provide initial screenings to determine if referrals are needed:
- School nurses screen for hearing and visual loss and refer those with positive outcomes
- Physician assistants and medical technicians screen for cognitive issues during the Medicare Annual Wellness Visit and refer those with positive outcomes
- Speech language pathologists screen for hearing loss and refer those with positive outcomes
- Dental technicians and assistants screen for high blood pressure and refer those with positive outcomes
In the same way, HCPs perform cognitive screenings on at-risk patients and refer those with positive outcomes. Medicare encourages health-related screenings by licensed healthcare professionals to get the patient to the correct provider as quickly as possible.10
To be clear, we (the authors) do not recommend cognitive screenings for every patient. We recommend cognitive screenings when there exists an elevated index of suspicion or when the patient is “at risk” for cognitive issues based on their specific history, consistent with the ACHIEVE study.11 A clinical assessment of cognitive ability can only be determined through cognitive diagnostic tests (not screeners) managed by psychiatrists, psychologists, neuropsychologists, neurologists, or others with expertise in cognition diagnostics.
Potential Cognitive Changes Associated with Amplification
It remains unclear as to whether hearing aid amplification offers a primary benefit to people with impaired cognitive ability. Applying group findings to an individual is not viable as that protocol does not consider unique psychological, emotional, educational, and socio-economic characteristics. Additionally, the specific hearing aid selected (prescription vs OTC, manufacturer, model, etc), the programmable parameters applied, the fitting protocols used such as best practice hearing aid fittings versus first fit protocols, earmold plumbing, length of adaptation, and more, present formidable outcomes-based analytical challenges.
Amieva et al reported the association between hearing loss, hearing aid use, and cognitive decline based on 3,670 people (ages 65+ years) followed for 25 years.12 They concluded “self-reported hearing loss is associated with accelerated cognitive decline in older adults; hearing aid use attenuates such decline.”
Livingston et al reported 12 potentially modifiable risk factors account for approximately 40% of dementia risk, and untreated hearing loss was the largest potentially modifiable risk factor (8.2% PAF).13
Glick and Sharma stated that beyond the typical communication benefits of hearing aids, clinical intervention with best-practice, premium amplification over six months may promote improved cortical organization and functioning and may provide cognitive benefit.14
With the ACHIEVE study, Lin, Pike, Albert et al found that for two primary groups (one group treated with hearing aid amplification, the other treated with excellent counseling) of younger people with fewer risk factors, hearing intervention had no effect on reducing cognitive decline within 3 years.9 However, for those at increased risk of cognitive decline, i.e., those who were older and demonstrated delayed degradation in thinking and memory and had more risk factors for cognitive decline, hearing aid amplification slowed the loss of thinking and memory abilities by 48% over 3 years.
Beck and Deal noted that although the primary ACHIEVE study report indicated no differences in the cognitive outcomes for the two younger primary groups, it can be argued that there was no “untreated” control group.15 Both applied strategies may have been equally beneficial/detrimental when compared to each other. However, we do not know how either/both groups would have performed if compared to a traditional control (untreated) group.
Myrstad, Engdahl, et al report hearing loss is strongly associated with future dementia.16 They reported 7,135 participants assessed for dementia and hearing, who were re-evaluated approximately 20 years later. The people with hearing loss demonstrated a moderate association with dementia in participants less than 85 years of age. The authors concluded that hearing loss is a risk for dementia.
Cantuaria, Pedersen, and Waldorff reported on 573,088 people.17 The authors noted a link between hearing loss and the development of dementia. They reported hearing loss was significantly associated with a 7% higher risk of dementia and stated hearing aid amplification “may prevent or delay the onset and progression of dementia.”
Sarant, Busby, Schembri, et al reported on 160 patients (49% female, mean age 73.5 years) with hearing loss who were fitted with hearing aids and a control group of 102 participants.18 Cognitive stability was witnessed in the hearing aid group, while the other group declined on working memory, visual attention, and psychomotor function. The authors concluded that hearing aid users demonstrated significantly better cognitive performance three years post-fitting and suggested hearing intervention may delay cognitive decline/dementia in older adults.
Searchfield, McAuliffe, Fok, et al report untreated hearing loss is a risk factor for age-related cognitive decline and that hearing aids have been shown to slow cognitive decline in a population at risk for dementia.19 They evaluated “Simple” hearing aid fittings (linear amplification with output limiting compression) versus a “Standard” hearing aid fitting approach (adaptive compression). They reported on 48 participants who completed assessments over 12 months. A statistically significant difference in fluid cognition scores was noted upon completion. Participants with the Simple fitting improved by 3.5 points; those with Standard fittings improved by 10.3 points. The authors reported hearing aid signal processing resulted in significantly different cognitive outcomes after 12 months and they indicated hearing aids improve cognition.
Choi, Adams, Crimmins, et al report that in the USA, frequent/regular hearing aid use by adults was related to decreased mortality risks despite age, hearing impairment, and demography.20 Hearing aid wearers had socioeconomic benefits, fewer medical comorbidities, and better access to healthcare, all of which likely contributed to decreased mortality risks.
Screen and Re-screen?
Some professionals have reported changes in cognitive screening results post-amplification. Further research is indicated to identify why or if a cognitive screener might demonstrate positive, stable, or negative changes over time. Additional information would help inform hearing care professionals’ use of cognitive screening for their patients.
Among the reasons cognitive screeners may indicate a change over time:
1. It is possible that the test-retest integrity of the selected cognitive screener test is wide. Specifically, at one point in time the patient may have tested on one end of the test-retest spectrum, and later, on the opposite end.
2 It is possible the patient has physically and cognitively aged significantly between the first and second test. For example, in a 36-year-old, six months of physical/cognitive change is often insignificant, depending on their comorbidities. However, a six-month change in an 81-year-old may be significant. These more rapid changes in older adults may be attributed to advanced diabetes, late-stage cancers, advanced metabolic disorders, advanced cardiac and pulmonary disorders, mild cognitive impairment, ADRD, and more.
3. It is possible that for people with hearing and listening difficulties, improving their ability to hear through amplification, or improving their ability to listen by managing the signal-to-noise ratio (SNR) allows them to better hear, understand, respond to, and make sense of the acoustic information around them. Specifically, availing better auditory sensory information (i.e., bottom-up) facilitates improved auditory processing (i.e., top-down) and potentially contributes to an improved cognitive screening result.
4. Miscellaneous reasons for improved cognitive screenings over time may include the patient wasn’t trying his/her best at the first or second test, the patient was distracted at either test, the cognitive screener was not a good screener, the interpretation of the cognitive screener results was subjective and possibly inaccurate as the person administering/scoring the test had an undiagnosed hearing or listening problem, perhaps the test administrator wasn’t thoroughly trained to administer the test, perhaps she incorrectly recorded the results, or perhaps that person was inattentive, distracted, inexperienced, and more.
5. It is possible that due to an improved sensory representation of the acoustic sound scene, the patient may have improved their cognitive ability over a six-month period.12
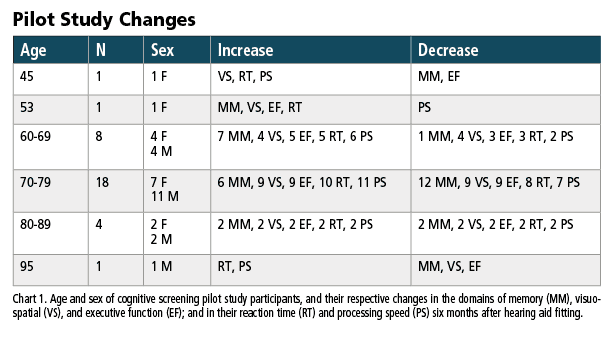
Pilot Study: Cognitive Screenings Pre- and Post-Hearing Aid Fitting
Recently, an office-based clinical evaluation and pilot study was undertaken to better understand what might happen if patients who received a cognitive screening and hearing aids were re-screened some six months after receiving hearing aid amplification and their first cognitive screening.
The data reported below only includes participants with mild-moderate sensorineural hearing loss (SNHL) and no known comorbidities. The total count includes 33 people with an average age of 73 years (15 females average age of 71 years, and 18 males average age of 75 years are included). Participant age ranges were two people ages 40-59, eight people ages 60-69, 18 people ages 70-79, four people ages 80-89, and one person age 95. The group pure tone average (PTA) was 41 dB for 66 ears (female left ear PTA 41 dB, female right ear PTA 42 dB, male left ear PTA 41 dB, male right ear PTA 40 dB).
The computerized in-office cognitive screener used was the Cognivue Thrive, which objectively screens and quantifies five factors including three primary domains: memory, visuospatial, and executive function, and two processing parameters (reaction time and processing speed). The Cognivue Thrive is an FDA registered cognitive screener. The assessment is self-administered by the participant, thereby eliminating subjective test administrator errors or biases, and requires only 5-7 minutes of test time.
5 Factors
The five factors screened and reported via Cognivue Thrive:
Domain 1: Memory (MM) Scores of 77-100 are considered good.
Domain 2: Visuospatial (VS) Scores of 59-100 are considered good.
Domain 3: Executive Function (EF) Scores of 75-100 are considered good.
Reaction Time (RT) < 900 msec is considered good.
Processing Speed (PS) <1900 msec is considered good.
As noted, the number of people in the pilot study is small. As such, detailed statistics and analysis are not encouraged as the data pool is too small to be representative or statistically meaningful across the reported decades. Nonetheless, when taken as a whole, some gross trends seem apparent. Specifically, the domains of memory, visuospatial, and executive function remain equivocal across six months. That is, just as many people improved their performance as those who performed worse over time.
As can be seen in Chart 1, 16 subjects improved their cognitive screening memory score and 17 decreased over the six months. Regarding visuospatial ability, 17 increased their cognitive screening memory score, 16 decreased. Regarding executive function, 17 increased their cognitive screening executive function score while 16 decreased. Nearly equivalent improvements and degradation was observed across the three primary domains, in this small sample.
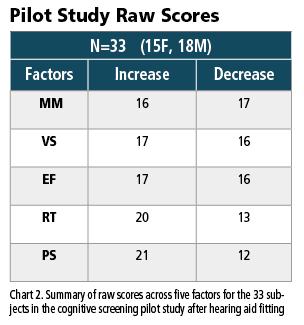
However, during the same six-month period, screening scores for processing speed and reaction time improved for 21 and 20 subjects (respectively) and decreased for 12 and 13 (respectively). Taken together, these equivalent domain changes and potential processing factor improvements (if truly present and repeated in larger studies in the future) may indicate that higher level domain changes remain stable (for the group) over time, while processing factors (for the group) may improve over time.
Discussion
This article has addressed multiple areas of discussion.
1. Through the literature review reported above, it seems clear that untreated hearing loss tends to exacerbate cognitive decline in older, at-risk adults.
2. Audiologists who have been trained to administer cognitive screening tests should do so for appropriate at-risk patients in accordance with AAA and ASHA Scope of Practice statements and should refer people with positive outcomes for further evaluation and treatment.
3. Although it is unclear as to whether hearing aid amplification offers a significant benefit to all people with impaired cognitive ability, multiple peer-reviewed articles (see above) demonstrate that cognitive and communication benefits secondary to hearing aid fittings are apparent for many people.
4. Significant and unique variables contribute to the individual’s outcome. Factors include psychological, emotional, educational, socio-economic, hearing or listening ability in quiet and noise, specific hearing aid selected (prescription vs OTC, manufacturer, model), best practice or other protocols, length of adaptation, comorbidities, type and degree of hearing loss, and more. In brief, although learnings and outcomes from large studies help us understand and predict trends for the population, the unique variables and outcomes for the individual are more difficult to predict.
5. We report a pilot study of 33 adults in which the gains and losses across the primary domains of executive function, memory, and visuospatial remained equivocal six months after hearing aid fittings. However, changes appeared to occur regarding improved speed of processing and reaction time. If repeated by others, these may be areas for future exploration.
The interest, interactions, and overlapping signs and symptoms between audiology and cognition, and the impact of amplification, continue to evolve. The authors remain grateful for the vast support shown by thousands of professionals who continue to read, write, investigate, and publish on these matters.
Douglas L. Beck, AuD, F-AAA, CCC-A, is among the most prolific authors in audiology, and a member of The Hearing Review editorial advisory board. His publications address a wide variety of audiology, clinical, academic, and professional topics. He can be reached at [email protected] or www.douglaslbeck.com.
Rhee Rosenman-Nesson, AuD, CCC-A, is the founder of Hearing Doctors of New Jersey. By applying the latest research along with cutting-edge technology, she focuses on treating hearing loss early to improve one’s overall health and cognitive function.
Laiba Ishtiaq, BA, is currently a second-year doctoral student in the Audiology program at Montclair State University in New Jersey and serves as the president of the Student Academy of Audiology (SAA) chapter at her university.
References
1. Reed NS, Garcia-Morales EE, Myers C, et al. Prevalence of Hearing Loss and Hearing Aid Use Among US Medicare Beneficiaries Aged 71 Years and Older. JAMA Netw Open. 2023;6(7):e2326320. Published 2023 Jul 3. doi:10.1001/jamanetworkopen.2023.26320
2. Manly JJ, Jones RN, Langa KM, et al. Estimating the Prevalence of Dementia and Mild Cognitive Impairment in the US: The 2016 Health and Retirement Study Harmonized Cognitive Assessment Protocol Project. JAMA Neurol. 2022;79(12):1242-1249. doi:10.1001/jamaneurol.2022.3543
3. American Speech-Language-Hearing Association. Scope of practice in audiology [Scope of Practice]. 2018. https://www.asha.org/policy/sp2018-00353/
4. American Academy of Audiology. Scope of practice. April 27, 2023. https://www.audiology.org/wp-content/uploads/2023/04/Scope-of-Practice_2023.pdf
5. Beck DL. Audition, Amplification, and Social Engagement. The Hearing Journal. 2024;77(1):18-21.
6. Shen J, Anderson MC, Arehart KH, Souza PE. Using Cognitive Screening Tests in Audiology. Am J Audiol. 2016;25(4):319-331. doi:10.1044/2016_AJA-16-0032
7. Kricos PB. Audiologic management of older adults with hearing loss and compromised cognitive/psychoacoustic auditory processing capabilities. Trends Amplif. 2006;10(1):1-28. doi:10.1177/108471380601000102
8. Arias JJ, Manchester M, Lah J. Direct to Consumer Biomarker Testing for Alzheimer Disease-Are We Ready for the Insurance Consequences?. JAMA Neurol. 2024;81(2):107-108. doi:10.1001/jamaneurol.2023.4811
9. Beck DL, Weinstein BE, and Harvey MA. Dementia Screening: A Role for Audiologists. Hearing Review. 2018;25(7):36-39. https://hearingreview.com/inside-hearing/research/dementia-screening-role-audiologists
10. Fifer R. CPT Codes, MAASA, Scope of Practice, Medicare, and More: An Interview with Robert Fifer, PhD. Hearing Review. 2022;29(5):28-31. https://hearingreview.com/inside-hearing/research/cpt-codes-maasa-scope-of-practice-medicare-and-more-an-interview-with-robert-fifer-phd
11. Lin FR, Pike JR, Albert MS, et al. Hearing intervention versus health education control to reduce cognitive decline in older adults with hearing loss in the USA (ACHIEVE): a multicentre, randomised controlled trial. Lancet. 2023;402(10404):786-797. doi:10.1016/S0140-6736(23)01406-X
12. Amieva H, Ouvrard C, Giulioli C, Meillon C, Rullier L, Dartigues JF. Self-Reported Hearing Loss, Hearing Aids, and Cognitive Decline in Elderly Adults: A 25-Year Study. J Am Geriatr Soc. 2015;63(10):2099-2104. doi:10.1111/jgs.13649
13. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission [published correction appears in Lancet. 2023 Sep 30;402(10408):1132]. Lancet. 2020;396(10248):413-446. doi:10.1016/S0140-6736(20)30367-6
14. Glick HA, Sharma A. Cortical Neuroplasticity and Cognitive Function in Early-Stage, Mild-Moderate Hearing Loss: Evidence of Neurocognitive Benefit From Hearing Aid Use. Front Neurosci. 2020;14:93. Published 2020 Feb 18. doi:10.3389/fnins.2020.00093