By Tish Ramirez, AuD; Caroline Hamiton, BSc (Audiology), MSc (Neuroscience); Sook Ling Leong, PhD
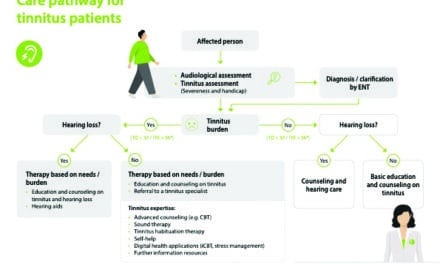
It is reported that 10-15% of the global adult population suffers from tinnitus 1-4, the chronic phantom auditory percept that has been attributed to abnormal firing patterns in the brain.4 According to the American Tinnitus Association (ATA), 45 million Americans reported experiencing tinnitus in the US Centers for Disease Control (CDC) National Health and Nutritional Examination Survey. Of these, nearly 20 million people are dealing with burdensome tinnitus on a regular basis, with approximately 2 million people struggling with severe, sometimes debilitating tinnitus.5 Among tinnitus sufferers, 90% also experience some degree of hearing loss.6 Additionally, tinnitus is the number one cause of disability among US veterans, according to the US Department of Veteran Affairs (VA), with some two million veterans receiving compensation for their tinnitus in fiscal year 2020.7
As hearing care professionals know, tinnitus is associated with a range of comorbidities, including psychological disorders that have a significant and multifaceted negative impact on health.8 Many tinnitus patients report feeling distressed by their symptoms and a resulting diminishment in their overall quality of life and that of their families.8 While there are several treatment approaches available, ranging from hearing aids with masking programs to cognitive behavioral therapy (CBT), tinnitus continues to be a major health issue in our society with limited treatment options that are consistently effective or accessible across sufferers.9-10
To address the unmet clinical need for a safe, effective, and scalable tinnitus treatment, Neuromod Devices Limited (Dublin, Ireland) developed a non-invasive bimodal (sound and tongue) stimulation portable device, Lenire® , that has been CE marked in Europe for use by tinnitus sufferers 18 years of age and older in order to alleviate the symptoms of chronic, subjective tinnitus. The Lenire treatment device (Figure 1) consists of a controller that connects to wireless consumer headphones for sound delivery to the ears and connects to a mouth component, known as a Tonguetip® that provides gentle electrical stimulation to the tongue surface. Users are recommended to use the device for 60 minutes daily (two 30-minute sessions consecutively or at different times of the day). Lenire is already commercialized across Europe and available by audiologists or hearing technicians in numerous hearing centers for treating tinnitus.

Figure 1. Neuromod Lenire treatment device. (a) Illustration of the Neuromod Lenire device during use. (b) Individual components of the Lenire device: handheld Controller; a Tonguetip Intra-Oral Device (IOD), which delivers gentle electrical stimulation to the tongue; and wireless consumer headphones that deliver audio stimulation.
Over the past five years, Neuromod Devices has completed two large-scale clinical trials to evaluate the efficacy of its propriety technology in more than 500 participants with tinnitus.11-12 This is the first of several Hearing Review articles relating to the Neuromod clinical studies. The primary purpose of this series is to review the clinical data, discuss general experiences, and considerations around treating tinnitus with Lenire, and consider how emerging therapies with bimodal stimulation may impact the future of tinnitus treatment. In this article, we will present and discuss the user experience of Lenire based on exit interviews from Neuromod’s first clinical trial referred to as TENT-A1 (Treatment Evaluation of Neuromodulation for Tinnitus-Stage 1, clinicaltrials.gov: NCT02669069).
TENT-A1 Design and Topline Outcomes
In 2020, results from TENT-A1, a large clinical investigation for bimodal stimulation in tinnitus were published in Science Translational Medicine.12 TENT-A1 was a randomized, double-blind, parallel-arm clinical trial that evaluated the efficacy and safety of 12 weeks of three bimodal stimulation parameter sets (PS1, PS2, and PS3), with post-treatment follow-up assessments at 6 weeks, 6 months, and 12 months in 326 enrolled tinnitus participants.
One key outcome measure for assessing improvements in tinnitus symptom severity used in this study was the validated patient-reported Tinnitus Handicap Inventory (THI).13 Results of TENT-A112revealed that all three investigated parameter sets are effective in reducing tinnitus symptoms during the treatment period. Arms 1 (PS1), 2 (PS2), and 3 (PS3) exhibited a statistically significant decrease of 14.6, 14.5, and 13.5 points in THI, respectively, from baseline to the end of the 12-week treatment period. There were no observed significant differences between arms during the treatment phase. Interestingly, exploratory analyses indicated that a majority of the benefit in tinnitus symptoms occurred after just six weeks of treatment. Furthermore, all three arms resulted in a high percentage of participants exhibiting improvements in tinnitus symptoms out to 12 months after the end of treatment. Importantly, there were no device related Serious Adverse Events (SAEs) in the study.
In TENT-A112, participants were advised to use the device daily for the 12-week treatment period. At the final visit, the device was returned, and the embedded log file was downloaded and recorded as part of the clinical trial dataset. Findings from the study showed that the tolerability of the treatment was found to be very high – 84% of participants were compliant to the treatment protocol (ie, used the device for the minimum compliance criterion of 36 hours), suggesting that a large proportion of the participants of the study were able to readily use the device on a routine basis.
During the final visit of the study, an exit interview was conducted to obtain qualitative feedback from participants about their experience during the clinical trial. The exit interview was a survey relating to the participant’s user experience with the device, the device’s overall performance during the investigation, and overall satisfaction with the device.
Participants consistently reported a high level of satisfaction with using the device, and they indicated that it improved their tinnitus symptoms and overall quality of life with minimal disruption. As noted in the published Science Translational Medicine manuscript12, out of 272 responses, 66.5% indicated that overall, they have benefitted from using the device, while 77.8% of the 270 responses indicated that if they knew someone with tinnitus, they would recommend that they try this treatment. This high percentage of participants who felt they benefitted from the treatment was echoed by the key THI outcome measure where most of the participants exhibited an improvement in THI score during treatment, ranging from 85.9% for Arm 1, 88.8% for Arm 2, and 83.7% for Arm 3.
It is noteworthy that the efficacy and safety of the Lenire treatment was repeatable in the follow-up TENT-A2 (Treatment Evaluation of Neuromodulation for Tinnitus-Stage 2, referred to as TENT-A2, clinicaltrials.gov: NCT03530306) trial. In TENT-A2, additional parameters sets were included, and outcomes were intended to build upon and further support the findings of TENT-A1.11 Neuromod Devices will soon publish the TENT-A2 results.
User Experience of Lenire in TENT-A1
In addition to published findings12 relating to participants’ satisfaction with the Lenire treatment, the TENT-A1 Exit Interview consisted of questions assessing the devices’ degree of usability, the impact it may have on users, and its effect on daily life. In the next section of this article, we present and discuss key findings of the utility and usability of the Lenire treatment device.
Portable and Feasible Treatment Regime
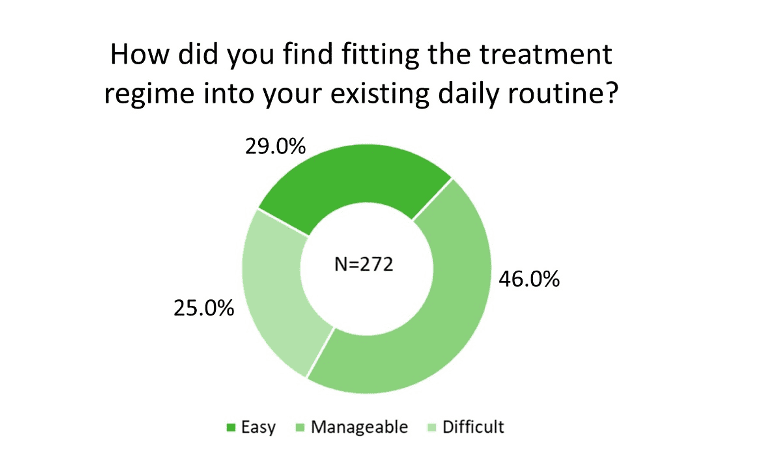
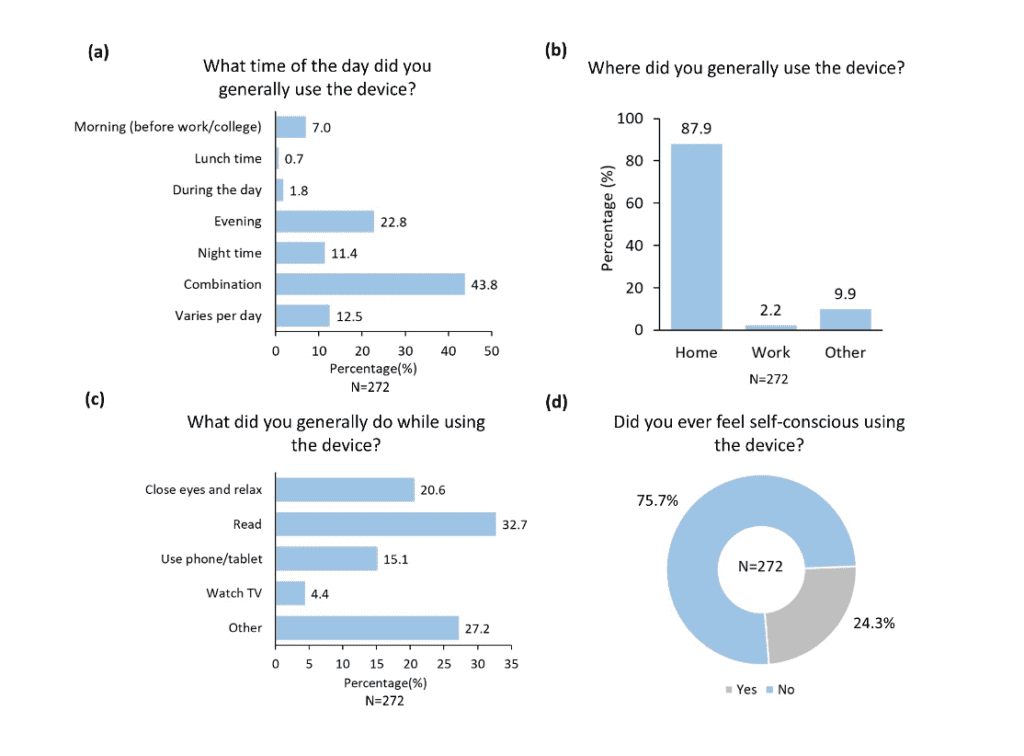
On the survey question of “How did you find fitting the treatment regime into your existing daily routine?,” of the 272 responses, 29.0% rated it “Easy” and 46.0% ranked it “Manageable” (Figure 2). The observation that participants used the device at various times throughout the day (Figure 3a), mostly at home (Figure 3b) while performing a variety of activities (Figure 3c) supports the versatility of Lenire. Importantly, 75.7% of the participants were not self-conscious when using the treatment device (Figure 3d). These findings suggest that participants could adapt the Lenire treatment to their daily lives, reinforcing one of the main concepts of this portable treatment device – to promote self-caring and autonomy of tinnitus patients.
Practical Device Components
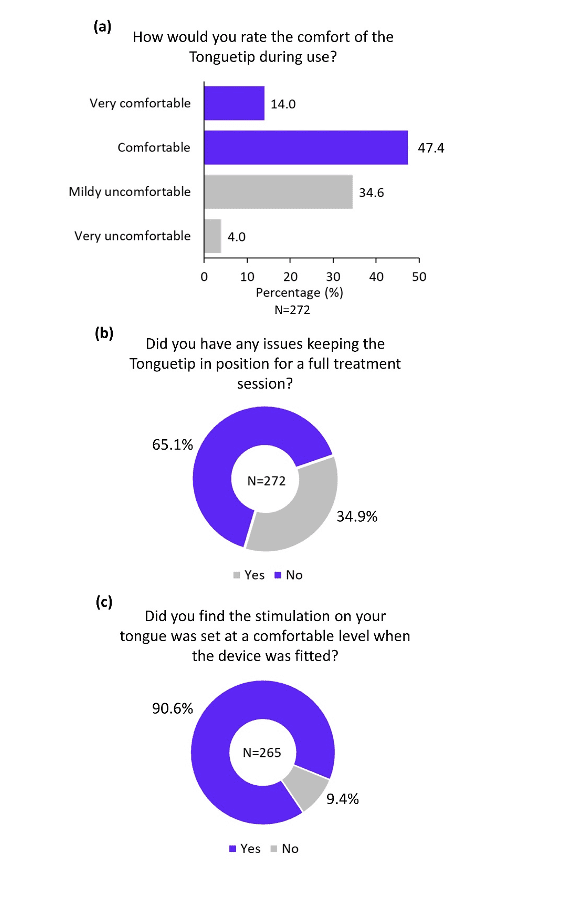
The overall practicality of the different components of the device was rated highly positive in the survey. More than 80% of participants reported that the headphones were comfortable (Figure 4a), and more than 90% of participants reported that the headphones were adjusted to a comfortable level during device fitting (Figure 4b). Similarly, the majority of participants found the Tonguetip comfortable and were able to keep it in position for a full treatment session (Figure 5a, b). More than 90% of participants reported that the stimulation on the tongue was set at a comfortable level during fitting (Figure 5c). Lastly, more than 90% of participants found the controller easy to use (Figure 6a), the feedback from the display lights understandable (Figure 6b), and that charging the controller was not an inconvenience (Figure 6c). These encouraging findings of the practicality of device components are of great importance indicating an alignment of the design of the device and actual real-word use.
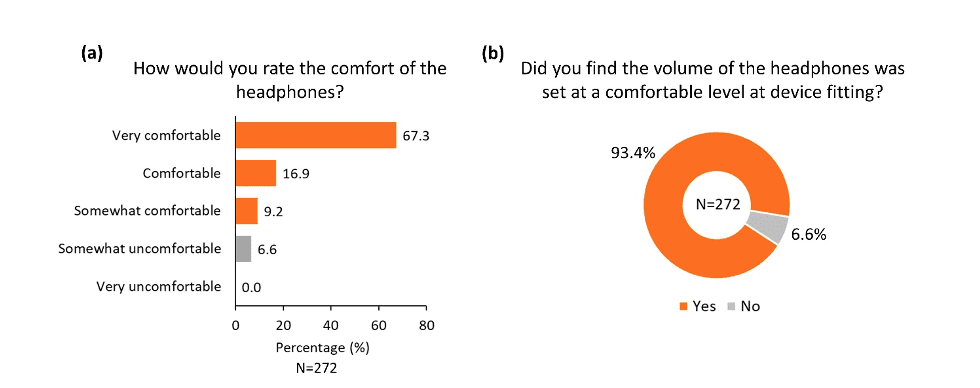
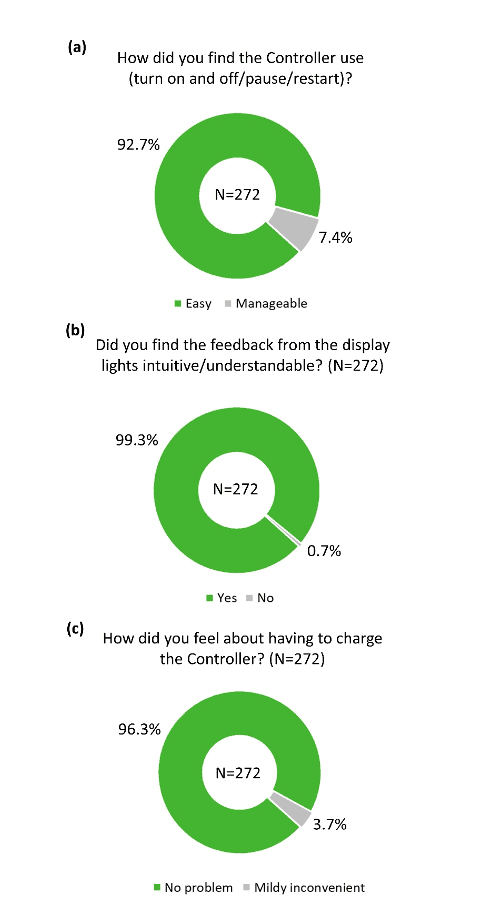
Suitability for Long-term Use and Multiple Daily Sessions
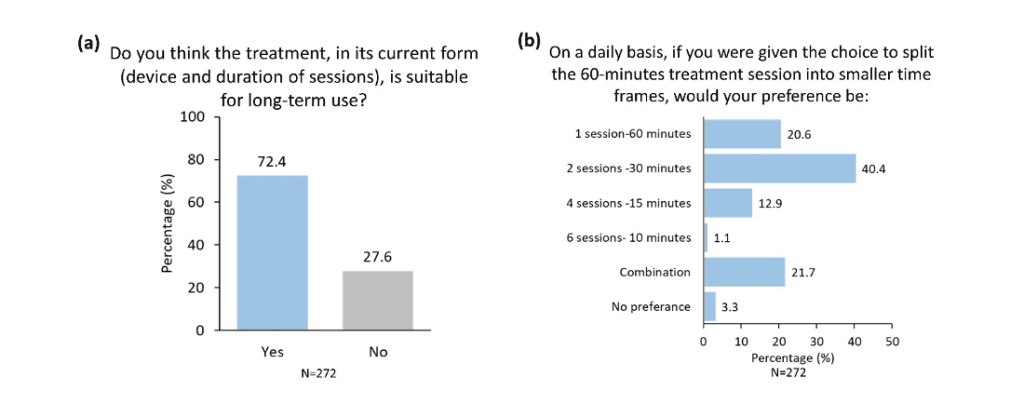
While a clear majority of participants (72.4%) reported that they think the current treatment is suitable for long-term use (Figure 7a), there was diversity in responses when asked about their preferred number of daily sessions (Figure 7b). Nevertheless, most participants (40.4%) selected the current recommended sessions as their preferred choice (Figure 7b). The knowledge that the current recommended treatment regimen in TENT-A1 is acceptable for most users is encouraging for the likelihood that future patients will properly implement the therapy and increase their chances of successful therapeutic outcomes.
On the whole, TENT-A1 study participants perceived the Lenire treatment device as highly practical with a treatment regime that can be fitted into their daily lives. The Lenire device offers tinnitus treatment in the privacy and comfort of the patient’s home environment and findings from this survey revealed that participants valued its portability and ease of use. Therapeutic sound and electrical stimuli are generated and controlled by the software embedded in the Controller component of the Lenire device. The Controller also serves as the patients’ interface to control its operation. Results presented in this article support that the design of the device is easy to use with user-friendly controls including START, STOP, PAUSE, sound stimulus VOLUME CONTROL, and electrical tongue stimulus INTENSITY CONTROL. Participants also found the Controller’s visual indicators while undergoing the treatment stimulation intuitive and understandable. Importantly, study participants were able to readily self-administer the Lenire device, with a large percentage of users benefiting from the treatment.



About the Authors: Tish Ramirez, AuD, is Chief Commercial Officer at Neuromod USA, Inc; Caroline Hamilton, BSc (Audiology), MSc (Neuroscience), is Audiology Director at Neuromod Devices in Dublin, Ireland; and Sook Ling Leong, PhD, is Clinical Research Manager at Neuromod Devices in Dublin, Ireland.
References
- Baguley D, McFerran D, and Hall D. Tinnitus. The Lancet. 2013;382(9904):1600-1607.
- Heller AJ. Classification and epidemiology of tinnitus. Otolaryngol Clin North Am. 2003;36(2):239-248.
- McCormack A, Edmondson-Jones M, Somerset S, Hall D. A systematic review of the reporting of tinnitus prevalence and severity. Hear Res. 2016;337:70-79.
- Moller AR, Langguth B, Ridder D, Kleinjung T, eds. Textbook of Tinnitus. SpringerLink; 2011.
- American Tinnitus Association (ATA) website. Understanding the Facts – Demographics. https://www.ata.org/understanding-facts/demographics. Accessed May 12, 2022.
- Hearing Loss Association of America (HLAA). Hearing Loss Facts and Statistics. https://www.hearingloss.org/wp-content/uploads/HLAA_HearingLoss_Facts_Statistics.pdf?pdf=FactStats. Accessed May 12, 2022.
- US Department of Veterans Affairs (VA). Veterans Benefits Administration Annual Benefits Report Fiscal Year 2020. https://www.benefits.va.gov/REPORTS/abr/docs/2020_ABR.pdf. Accessed May 12, 2022.
- Langguth B, Kreuzer PM, Kleinjung T, De Ridder D. Tinnitus: causes and clinical management. Lancet Neurol. 2013;12(9):920-930.
- Cima RFF, Maes IH, Joore MA, et al. Specialised treatment based on cognitive behaviour therapy versus usual care for tinnitus: A randomised controlled trial. The Lancet. 2012;379(9830):1951-1959.
- Tunkel DE, Bauer CA, Sun GH, et al. Clinical practice guideline: Tinnitus. Otolaryngol Head Neck Surg. 2014;151(2 Suppl):S1-S40.
- Conlon, B , Hamilton C, Hughes S, et al. Noninvasive bimodal neuromodulation for the treatment of tinnitus: Protocol for a second large-scale double-blind randomized clinical trial to optimize stimulation parameters. JMIR Res Protoc. 2019;8(9):e13176.
- Conlon B, Langguth B, Hamilton C, et al. Bimodal neuromodulation combining sound and tongue stimulation reduces tinnitus symptoms in a large randomized clinical study. Sci Transl Med. 2020;12(564):eabb2830.
- Newman CW, Jacobson GP, Spitzer JB. Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg. 1996;122(2):143-148.